Why use CanPath Data?
CanPath is Canada’s largest population health cohort and a national platform for population-level health research.
It is a unique Canadian platform allowing scientists to explore the complex factors that contribute to disease. It is a deeply characterized cohort of individuals who have provided broad consent and now include two per cent of all Canadians between 30 and 74 years of age.
CanPath can save researchers time — sometimes up to a decade — associated with arranging and measuring their own population samples.
Researchers around the world can readily integrate CanPath data into their own studies. The standardization and harmonization of data across CanPath’s regional cohorts has been facilitated by Maelstrom Research.
Check out our YouTube video on the CanPath Access Process to find out how you can access longitudinal health data and biosamples from more than 330,000 participants!
Data Highlights:
Datasets
All CanPath participants completed a detailed questionnaire at the time of recruitment (baseline) and continue to provide updated health and lifestyle information through follow-up questionnaires.
Nationally harmonized datasets include data collected by the five mature cohorts: BC Generations Project, Alberta’s Tomorrow Project, Ontario Health Study, CARTaGENE and the Atlantic PATH. Data from the Manitoba Tomorrow Project will be made available once participant recruitment is complete.
Harmonized datasets available include:
- Baseline Health and Risk Factors Questionnaire
- Baseline Health and Risk Factors Questionnaire with Additional Diseases
- Baseline Mental Health Questionnaire
- Baseline Physical Measures
- Follow-up Health and Risk Factors Questionnaire
- Pre-analytical Data Related to Biological Samples
- Genotyping Data
- CANUE Environmental Exposure Data
- COVID-19 Questionnaire – Now Available
Biosamples
CanPath has biological samples available for researchers, enabling wider and deeper investigations into the causes of cancer and chronic diseases. Biological samples were donated near the time of participant enrollment or during subsequent collection campaigns.
Available biosamples include serum, plasma, DNA, red blood cells, cryo-preserved whole blood, and urine samples.
Behind this national resource is a dedicated team with extensive expertise in biobanking. CanPath’s National Biosample Coordinator, Treena McDonald, ensures that samples are stored and managed according to international best practices. She is a member of the International Society for Biological and Environmental Repositories (ISBER), bringing global standards and over 15 years of biobanking experience to CanPath.
The Biosamples Standing Committee provides sample counsel and input that have impact at the pan-Canadian level, as well as enhancing coordination and best practice sharing across the regional cohorts. It ensures that biosamples are collected, processed, and stored in each CanPath region according to a core set of operating procedures that reflect as much consistency across the Regions as possible. The committee is led by Dr. Peter Watson, Director of Biobanking and Biospecimen Research Services, staff pathologist and senior scientist with BC Cancer, and Professor of Pathology at the University of British Columbia and adjunct professor at the University of Victoria.
Browse Portal
Access Process & Criteria
Requests for access to CanPath data and biosamples are accepted and reviewed throughout the year. The requirements for each review process are outlined below.
Access applications can be made directly through the CanPath Portal.
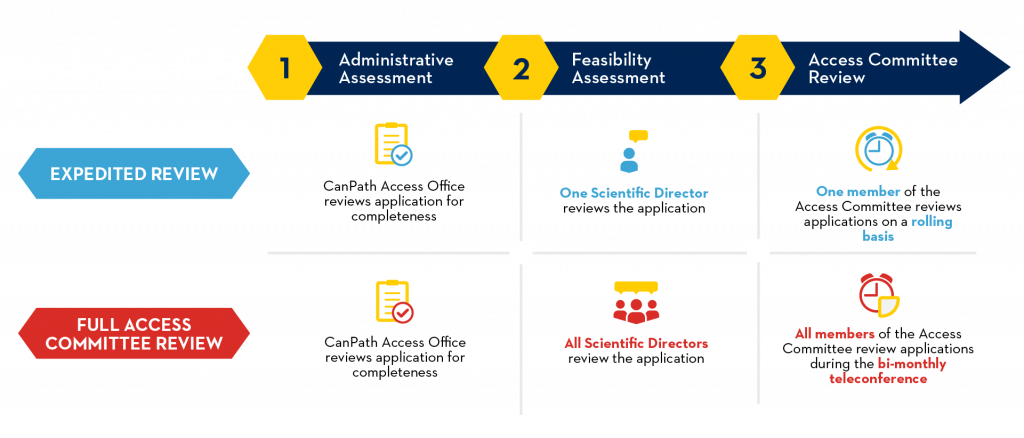
Access Criteria & Requirements
All completed and submitted Access Application Forms and associated documentation are reviewed by the independent Access Committee according to the following criteria:
- The Applicant is a bona fide researcher (i.e. evidence that the researcher has relevant experience and qualifications);
- The research project is in conformity with both the Guiding Principles of CanPath and the informed consents signed by the Research Participants (see Sections 2 and 4a of the CanPath Access Policy);
- The Access Office has provided proof of administrative completeness and availability of CanPath Data and/or Biosamples
The Access Office assessment has established that the application meets the following requirements:
- The research study has been deemed scientifically sound;
- The existence of adequate resources to effectively complete the research project has been established (e.g. funding, collaborators and staff);
- Sufficient justification for the need for the CanPath Data and/or Biosamples requested has been provided;
- The provision of the requested biosamples is justified based on the assessment of the value of returned data, the scientific contribution of the research project, the potential impact of providing the samples on future needs for the biosamples and the risk of sample depletion.
Expedited Review Criteria
Access Applications meeting the criteria for an Expedited Review are accepted on an ongoing basis and will be completed within approximately 4 months. This timeline is contingent on the applicant’s responsiveness throughout the process.
As noted in the CanPath Access Policy, the following criteria are used to determine if applications qualify for an Expedited Review:
- Requesting Harmonized Datasets;
- Low reputational risk based on the research question being addressed and its scientific merit;
- The project has been evaluated through a recognized scientific review or peer review process;
- There is evidence of financial support for the project; and
- The Research Team has sufficient membership and expertise to complete the analyses.
Note: With the exception of the first two points, applications do not need to meet all of these criteria to be considered for Expedited Review.
Full Access Committee Review Criteria
As noted in the CanPath Access Policy, Access Applications meeting any of the following criteria will require a Full Access Committee Review:
- Requests for data other than datasets listed as ‘Harmonized Data’;
- Access to biosamples are requested;
- Research question addresses a potentially contentious research question (e.g., compares outcomes by ethnicity or community, potentially negative impact on subsets of participants) or with a high risk of reidentification; or
- Request includes linkage to administrative health data.
Access Applications requiring a Full Access Committee Review are accepted on an ongoing basis.
Expedited COVID-19 Data Access
National harmonized data from the CanPath COVID-19 Questionnaire is now available to researchers. Given the immediate need for pandemic research, CanPath has revised its expedited review process to provide researchers timely access to the COVID-19 data. Requests for access to the national COVID-19 Questionnaire dataset will be eligible for Expedited Review.
Access Cost
CanPath has a cost recovery access fees model suited to the different budgets of trainees, early-career researchers, and established researchers. Applicants are invited to complete a Cost Estimate Form and submit by email to apply@canpath.ca.
Access Committee
The Access Committee reviews and makes approval decisions on research applications submitted for CanPath Data and/or Biosamples. The committee is composed of independent members from across Canada who have expertise in such relevant fields as biostatistics, epidemiology, and genomics.
Apply Now
If you would like to apply to access biosamples and linked administrative data for the same project, please contact the Access Office.
Questions?
Contact CanPath Access Officer, Nouar Elkhair